Bisphosphonates (BPs) are used to treat various bone diseases
Bisphosphonates (BPs) are used to treat various bone diseases. Bisphosphonates (BPs) are a new class of drugs used in various bone diseases and calcium metabolism diseases. It can specifically combine with hydroxyphosphatidite in bone to inhibit osteoclast activity, thereby inhibiting bone resorption. It is used to treat osteoporosis, osteitis deformans, hypercalcemia and bone pain caused by bone metastasis of malignant tumors.
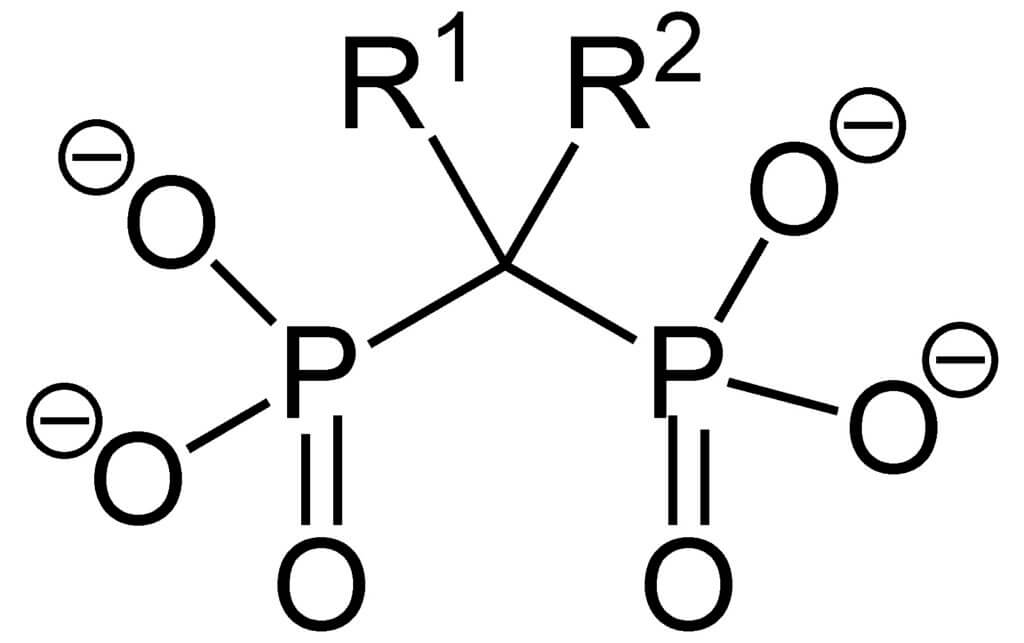
All bisphosphonate drugs share a common phosphorus-carbon-phosphorus “backbone”:
The two PO 3 (phosphonate) groups covalently linked to the carbon determine the name of the “bisphosphonate” and the function of the drug. Bis refers to the fact that there are two such groups in the molecule.
- Long side chain (R2 in the figure) determines the chemical properties, mode of action and strength of bisphosphonate drugs.
- Short side chain (R1) is usually called “hook” and mainly affects the chemical properties and pharmacokinetics.
More than 30 years ago, Fleisch et al. discovered that pyrophosphate, which exists in plasma and urine, can inhibit ectopic calcification. However, the oral administration of pyrophosphate is ineffective, and the injection administration is rapidly inactivated by enzymatic hydrolysis. Later studies have found that replacing the POP group in the pyrophosphate structure with PCP groups can change the physical and chemical properties of pyrophosphate and increase its resistance. The stability of hydrolase changes its biological properties and toxicological effects. Subsequently, a series of bisphosphonate compounds were synthesized.
Among them, the first-generation etidronate sodium was first listed in 1987 by Proter & Gamble of the United States. Later, the second generation of clodronate, pamidronate and tiludronate and the third generation of alendronate, neridronate and opadronate were successively developed. (olpadronate), risedronate sodium and ibandronate sodium and so on.
The structure-activity relationship of bisphosphonates is not yet fully understood. But it has been clear that its basic structure P-C-P is a necessary condition for activity.
The strength of each drug depends on the type of side chain substituted on the C atom. For example, clodronate in which R1 and R2 are replaced by Cl atoms, its anti-bone resorption strength is 10 times that of etidronate.
For example, R2 is an -OH group, R1 is a side chain substitution containing N atoms, and its effect is greater. Pamidronate and alendronate are 100 times and 1,000 times stronger than etidronate sodium, respectively; R1 Ibandronate sodium, which is obtained by adding methyl and pentyl groups to the N atom of the side chain, is 10,000 times more active than etidronate sodium.
Substitution of the H atom on R1 by picolinyl can also increase the anti-bone resorption effect. For example, the strength of risedronate sodium is 5000 times that of etidronate sodium.