New crystal phase found in calcium phosphate family
New crystal phase found in calcium phosphate family. Calcium phosphate (calcium orthophosphate) is widely found in nature and organisms, and it is a commonly used biomedical material.
The calcium phosphate family contains a variety of phases, but based on different properties, the biological effects and application directions of various phases in vivo are also different.
For example, the main inorganic component of vertebrate bones and teeth is hydroxyapatite, but its precursor phase is considered to be amorphous calcium phosphate, and the intermediate phase is calcium hydrogen phosphate dihydrate or octacalcium phosphate; the components of pathological mineralization include Hydroxyapatite, calcium hydrogen phosphate, calcium pyrophosphate, etc.; in biological applications, hydroxyapatite is a component of the surface coating of common orthopedic plants, and the high-temperature sintered bioceramics are mainly tricalcium phosphate, calcium-phosphorus-based bone The hydration product of cement is often calcium hydrogen phosphate dihydrate.
The crystalline phase of calcium phosphate was basically discovered before the 20th century (amorphous phase was discovered in the 1950s) and accurately characterized in the 20th century, and then there is no typical (undoped, non-substituted, only containing Ca, P, O, H) New crystal phase report. In addition, there are only two types of calcium phosphate with a molar ratio of calcium to phosphorus of 1:1:
- Calcium hydrogen phosphate dihydrate (brushite, CaHPO4•2H2O, monetite phase, we jokingly call it “Dabao”)
- Anhydrous calcium hydrogen phosphate (monetite, CaHPO4, monetite phase),
The crystal phase containing a crystal water has not yet been discovered.
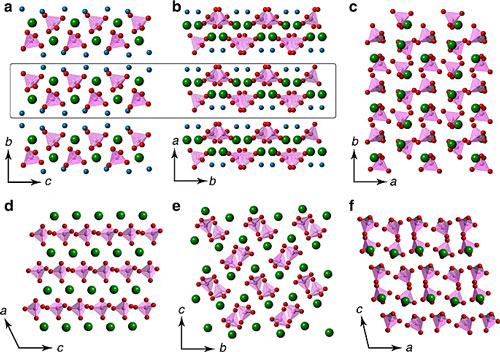
In 2020, a collaborative team from Shanghai Institute of Ceramics, Chinese Academy of Sciences, University of Hannover, Germany, Stockholm University, Sweden, and Curtin University, Australia, controlled a special amorphous calcium phosphate (molecular formula CaHPO4•xH2O, ACHP) The conversion of the water environment (water/methanol mixed solvent, or humid air) is very lucky to obtain the crystal phase product of calcium hydrogen phosphate monohydrate (CaHPO4•H2O, DCPM).
This discovery not only allowed the calcium phosphate family to welcome new members nearly a century later.
