Organophosphate
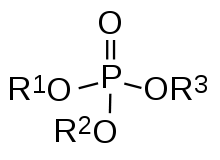 Organophosphate Flame retardant , also known as orthophosphate (to distinguish it from phosphite) or Phosphate ester, is an ester derivative of phosphoric acid and belongs to a category of phosphoric acid derivatives. Phosphoric acid is a tribasic acid, so according to the number of substituted hydrocarbon groups, phosphate can be divided into primary phosphate (monophosphate, hydrocarbyl phosphate), secondary phosphate (phosphodiester) and tertiary phosphate (triester of phosphate) .
Organophosphate Flame retardant , also known as orthophosphate (to distinguish it from phosphite) or Phosphate ester, is an ester derivative of phosphoric acid and belongs to a category of phosphoric acid derivatives. Phosphoric acid is a tribasic acid, so according to the number of substituted hydrocarbon groups, phosphate can be divided into primary phosphate (monophosphate, hydrocarbyl phosphate), secondary phosphate (phosphodiester) and tertiary phosphate (triester of phosphate) .
How to make Organophosphate?
First, let phosphoric acid esterificate: Condensation of 1 mol phosphoric acid with 1, 2, 3 mol alcohols to generate phosphoric acid monoester, diester and triester
OP(OH)3 + ROH → OP(OH)2(OR) + H2O
OP(OH)2(OR) + R’OH → OP(OH)(OR)(OR’) + H2O
OP(OH)(OR)(OR’) + R”OH → OP(OR)(OR’)(OR”) + H2O
Organophosphate as Flame Retardant of Lithium-ion Battries
Compared with metal lithium batteries, lithium-ion batteries have higher safety, but they will still explode and catch fire under extreme conditions such as overcharging, overheating, and squeezing.
At present, the development of vehicle power batteries puts forward higher safety requirements for lithium-ion batteries. Since lithium ion batteries use flammable organic electrolyte, the thermal stability of the electrolyte is very important to the safety of the battery.
Studies have shown that trimethyl phosphate (TMP), dimethyl methyl phosphate (DMMP) and tetraisopropyl methylene diphosphate (TPPP) three alkyl phosphates have an effect on the thermal stability of electrolytes commonly used in lithium-ion batteries. Influence, found that DMMP has the highest flame retardant ability.
