2021 Nobel Prize in Chemistry: 2021 Nobel Prize Chemistry: Revolution on molecules synthesizing. They have revolutionized the way humans synthesize molecules.
At about 17:50 Beijing time on October 6, 2021, German scientist Benjamin List (Benjamin List) and American scientist David MacMillan (David MacMillan) “developed asymmetric organic catalysis” Won the 2021 Nobel Prize in Chemistry.
Winners of the 2021 Nobel Prize in Chemistry: German scientist Benjamin List and American scientist David MacMillan
Benjamin List, the director of the Max-Planck-Institut für Kohlenforschung, a member of the German Academy of Sciences. Lister was born in Frankfurt, Germany in 1968 and received his doctorate from University Frankfurt in 1997. In 1999, he worked as an associate professor at the Scripps Research Institute in the United States, and then joined the Max Planck Institute for Coal. Lister currently serves as an honorary professor at the University of Cologne, Germany. He was awarded the Thomson Reuters Citation Laureate in 2009 and the Gottfried Wilhelm Leibniz-Prize in 2016.
 David MacMillan is a professor of chemistry at Princeton University and a member of the National Academy of Sciences. Macmillan was born in Bellshill, Scotland, England in 1968. He received his Ph.D. from the University of California, Irvine in 1996, and then entered Harvard University for postdoctoral research. From 1998 to 2006, he taught at the University of California, Berkeley and California Institute of Technology. Joined Princeton University in 2006. He has won the Royal Society of Chemistry Corday-Morgan Medal in 2004, Harrison Howe Award in 2015, Ryoji Noyori Award in 2017 and many other awards in the field of chemistry. He is also the founding editor-in-chief of the famous journal Chemical Science under the Royal Society of Chemistry.
David MacMillan is a professor of chemistry at Princeton University and a member of the National Academy of Sciences. Macmillan was born in Bellshill, Scotland, England in 1968. He received his Ph.D. from the University of California, Irvine in 1996, and then entered Harvard University for postdoctoral research. From 1998 to 2006, he taught at the University of California, Berkeley and California Institute of Technology. Joined Princeton University in 2006. He has won the Royal Society of Chemistry Corday-Morgan Medal in 2004, Harrison Howe Award in 2015, Ryoji Noyori Award in 2017 and many other awards in the field of chemistry. He is also the founding editor-in-chief of the famous journal Chemical Science under the Royal Society of Chemistry.
Nobel Prize official website: https://www.nobelprize.org/
Official interpretation of the Nobel Prize: Their tools revolutionized molecular synthesis
Chemists can connect small chemical building blocks together to create new molecules, but it is very difficult to control invisible chemical substrates and make them combine in the desired way.
Benjamin Lister and David Macmillan won the 2021 Nobel Prize in Chemistry for developing a new and ingenious molecular construction tool called organocatalysis.
This tool can not only be used to develop new drugs, but also It can also make chemistry more environmentally friendly.
Many areas of industry and academia depend on the ability of chemists to construct new functional molecules.
These new molecules can be used in any field: capture light in solar cells, or play a role in energy storage in batteries; they can be used to make light running shoes, and they can also inhibit disease processes in the body.
However, if we compare nature’s ability to generate chemical reactions with our own capabilities, we will find that humans are stagnant as if they have been in the Stone Age.
Evolution has created extremely special tools-enzymes, used to build molecular complexes that give life shape, color and function.
When chemists first separated these masterpieces of chemistry, they could only look at evolution with admiration, and the hammers and chisels in their own toolboxes for building molecules were blunt and unreliable.
Therefore, when they copy the products of nature, they often get many unwanted by-products.
New tools for fine chemistry
Every new tool that a chemist adds to the toolbox improves the precision with which we construct molecules. Scientists very slowly but practically developed chemistry from chiseling stone to a more refined craft. This is of great benefit to mankind, and some of these tools have won the Nobel Prize in Chemistry.
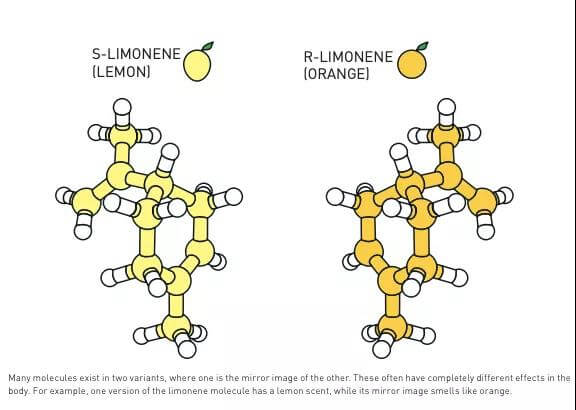
Figure 2: Many molecules exist in two variants, one of which is a mirror image of the other, and they usually have completely different effects on the human body. For example, one of the limonene molecules has a lemon scent, while the mirror image molecule tastes like an orange.
The discoveries commended by the Nobel Prize in Chemistry in 2021 have brought human construction of molecules to a whole new level.
It not only makes chemistry more environmentally friendly, but also makes it easier to synthesize asymmetric molecules.
In the process of constructing compounds, we often get two molecules whose structures are mirror images of each other, just like our hands.
Chemists usually only want one of them—especially when producing drugs, but it is difficult for them to find an effective method.
The “Asymmetric Organocatalysis” developed by Benjamin List and David Macmillan is simple and wonderful. In fact, many people wonder why we didn’t think about it earlier.
Why on earth? This is not an easy question to answer, but before we try, we need a quick review of history. We will define the terms “catalysis” and “catalyst” to pave the way for the 2021 Nobel Prize in Chemistry.
Catalyst: accelerate chemical reaction
In the 19th century, when chemists began to explore the ways in which different chemical substances react with each other, they made some strange discoveries.
For example, if they put silver in a beaker containing hydrogen peroxide (H2O2), the hydrogen peroxide will suddenly start to decompose into water (H2O) and oxygen (O2), but the silver that triggers this process seems to be completely unreacted Impact.
Similarly, a substance obtained from sprouted grains can break down starch into glucose.
In 1835, the famous Swedish chemist Jacob Berzelius discovered the law in it. In an annual report of the Royal Swedish Academy of Sciences describing the latest developments in physics and chemistry, he described a new type of “force” that can “produce chemical activity.” He cited a number of examples in which a substance can start a chemical reaction as long as it is “present”, indicating that this seems to be a much more common phenomenon than previously imagined. He believes that the substance has a catalytic force and calls this phenomenon itself catalysis.
Catalyst brings plastic, perfume and delicious food
Since Bezelius, countless streams of water have passed through the reactors of experimental chemists, and they have discovered many catalysts that can break down molecules or assemble them together. Thanks to these catalysts, people can now produce thousands of different substances used in daily life, such as medicines, plastics, perfumes and flavors. In fact, it is estimated that 35% of the total global GDP is related to chemical catalysis in some way.
In principle, before the 21st century, all the catalysts we found fall into two categories. They are either metals or enzymes. Metals are generally excellent catalysts because they have a special ability to temporarily hold electrons during chemical processes or donate electrons to other molecules. This helps loosen the bonds between atoms in the molecule. The originally strong chemical bonds may be broken and new bonds may be formed.
However, the problem with some metal catalysts is that they are very sensitive to oxygen and water, so they can only function in the absence of oxygen and water, which is difficult to achieve in large-scale industries. In addition, many metal catalysts are heavy metals, which are harmful to the environment.
The catalytic process of life is extremely delicate
The second category of catalysts consists of a protein called enzyme. There are thousands of different enzymes in all organisms to drive the chemical reactions necessary for life.
Many enzymes are experts in asymmetric catalysis, and in principle they can always catalyze the production of one of the two mirror images.
Enzymes also always work together. When one enzyme completes the reaction, the other enzyme will take over the subsequent reaction. In this way, enzymes can construct complex molecules such as cholesterol, chlorophyll, or the toxin called strychnine (also known as strychnine) with amazing precision-this is the best we know.
One of the complex molecules (we will talk about it later).
Because enzymes are such effective catalysts, researchers in the 1990s tried to develop new enzyme variants to drive the chemical reactions required by humans. One of the teams came from the Scripps Research Institute in southern California, USA, and was led by the late Carlos F. Barbas III, who was behind this year’s Nobel Prize in Chemistry. Benjamin List was a postdoc in Barbas’s team when the brilliant idea was born.
Benjamin Lister thinks outside the box
Benjamin List’s research object is the catalytic antibody. Generally speaking, antibodies will attach to foreign viruses or bacteria in the human body. But scientists at the Scripps Research Institute redesigned them to enable them to drive chemical reactions.
In the process of researching catalytic antibodies, Benyamin started thinking about the actual working methods of enzymes. Enzymes are usually large molecules composed of hundreds of amino acids.
In addition to these amino acids, a considerable part of the structure of enzymes contains metals that help drive chemical processes. But the key to the problem lies here.
Many enzymes do not rely on the help of metals to catalyze chemical reactions.
On the contrary, the reaction is driven by one or a few individual amino acids in these enzymes.
Benyamin jumped out of the existing thinking and asked a question: Do amino acids have to be part of enzymes in order to catalyze chemical reactions? Can a single amino acid, or other similar simple molecules, also play the same role?
Revolutionary results are born
Benjamin knew that since the early 1970s, there have been studies using an amino acid called proline as a catalyst-but that was at least 25 years ago. If proline is really an effective catalyst, someone should continue to study it, right?
Benjamin thinks more or less this way: no one continues to study this phenomenon because it is not very effective. Without real hope, he tested whether proline can catalyze the aldol condensation reaction of the carbon atoms of two different molecules. Surprisingly, this simple attempt was immediately successful.
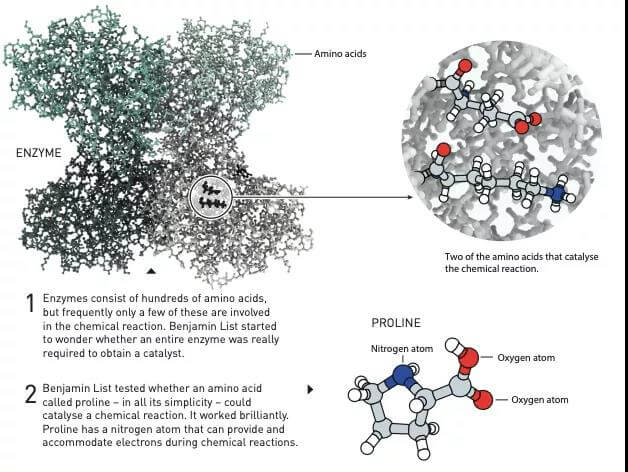 Figure 3: 1- Enzyme is composed of hundreds of amino acids, but only a few of them are related to chemical reactions. Benjamin List began to think about whether to obtain a catalyst, a complete enzyme must be required?
Figure 3: 1- Enzyme is composed of hundreds of amino acids, but only a few of them are related to chemical reactions. Benjamin List began to think about whether to obtain a catalyst, a complete enzyme must be required?
2-Benjamin Lister tested whether an amino acid called proline can catalyze chemical reactions. Although simple, proline is very effective in catalysis. A nitrogen atom of proline can provide or hold electrons in a chemical reaction.
Benjamin Lister found the way to the future
Benjamin List’s experiment not only proved that proline is an effective catalyst, but also proved that this amino acid can drive asymmetric catalysis: in the two mirror image isomers of a molecule, One of the isomers is a more common catalytic product than the other.
Unlike the researchers who tested proline catalysts before, Benjamin Lister understood the great potential of proline. Compared with metals and enzymes, proline is a coveted tool for chemists.
It is a very simple, cheap and environmentally friendly molecule. When publishing this discovery in February 2000, Lister described the asymmetric catalysis of organic molecules, a new concept with great potential: “The design and screening of these catalysts is one of our future goals.”
However, he is not alone in doing these studies. In a laboratory in northern California, David Macmillan is also working towards the same goal.
David Macmillan gave up the “squeaky” metal
Two years before the above story happened, David Macmillan left Harvard University and joined the University of California, Berkeley. At Harvard, he worked on the use of metals to improve asymmetric catalysis.
This field has attracted the attention of a large number of researchers, but David Macmillan discovered that the metal catalysts developed are rarely used in industry.
He began to think about the reasons and determined that the application of sensitive metals was too complicated and expensive.
It is relatively easy to achieve the oxygen- and water-free conditions required for certain metal catalysts in the laboratory, but it is very complicated to achieve such conditions in large-scale industrial production.
He concluded that he needed to rethink whether the chemical tools he was developing were useful. Therefore, when he joined Berkeley, he left the metal behind.
Develop simpler catalyst formats
In contrast to metals, David Macmillan began to develop a simple organic molecule. It can temporarily provide or hold electrons like metal. Here we need to define organic molecules—in short, the constituent molecules of all living things.
Organic molecules have a stable carbon skeleton, and active chemical groups are attached to the carbon skeleton, usually containing oxygen, nitrogen, sulfur or phosphorus.
Organic molecules are composed of simple common elements, but depending on the way they are composed, they may have complex properties.
David Macmillan’s knowledge of chemistry told him that for an organic molecule to catalyze the reaction he was interested in, it must be able to form imine ions. It contains nitrogen atoms and has an affinity for electrons.
He selected several organic molecules with the desired properties and tested their ability to drive the Diels-Alder reaction. Chemists use this reaction to build a ring of carbon atoms. As he expected and believed, the catalysis of these molecules is excellent. Some organic molecules also perform well in asymmetric catalysis. Among the two possible mirror image heterogeneous products, one of them accounts for more than 90%.

Figure 4: 1- The metal catalyst studied by David Macmillan is easily destroyed by humidity. He began to think about the possibility of developing a more durable catalyst.
2- He designed some simple molecules capable of generating imine ions, one of which was proven to perform well in asymmetric catalysis.
The birth of the term “organocatalysis”
When David Macmillan was about to publish the results, he realized that the catalytic concept he had discovered needed a name. In fact, researchers have successfully used small organic molecules to catalyze chemical reactions, but these are isolated cases, and no one realizes that this method can be promoted.
David Macmillan wanted to find a term to describe the method so that other researchers could understand that there are more organic catalysts waiting to be discovered. His choice is organocatalysis.
In January 2000, just before Benjamin List published his findings, David Macmillan submitted his manuscript to the scientific journal. He announced in the introduction of the paper: “Here, we have introduced a new organic catalysis strategy, and we expect that this strategy will be applicable to a series of asymmetric transformations.”
Organic catalysis is widely used
Benjamin Lister and David Macmillan independently discovered a completely new concept of catalysis. Since 2000, the development of this field is almost like a gold rush, in which Lister and Macmillan have maintained their leading positions. They designed a large number of cheap and stable organic catalysts that can be used to drive a variety of chemical reactions.
Organic catalysts are not only usually composed of simple molecules, but in some cases can also be applied to assembly lines, just like natural enzymes. In the past, in the chemical production process, it was necessary to separate and purify each intermediate product, otherwise too many by-products would be produced. This leads to the loss of some substances in each step of chemical synthesis.
The tolerance of organic catalysts is much higher, because relatively speaking, in more cases, several steps in the production process can be executed continuously. This is called a cascade reaction and can significantly reduce waste in chemical manufacturing.
Strychnine synthesis efficiency increased by 7000 times
An example of how organic catalysis makes chemical synthesis more efficient is the synthesis of naturally occurring and extremely complex strychnine molecules. Many people know Strychnin from the book “Queen of Murder Novels” Agatha Christie (Agatha Christie). However, for chemists, strychnine is like a Rubik’s Cube: a challenge you want to solve in as few steps as possible.
When strychnine was first synthesized in 1952, it required 29 different chemical reactions, and only 0.0009% of the starting material was synthesized into strychnine. The rest is wasted.
By 2011, researchers were able to use organic catalysis and cascade reactions to synthesize strychnine in only 12 steps, and the efficiency of the production process was increased by 7000 times.
The important position of organic catalysis in drug production
Organic catalysis has had a major impact on drug research that often requires asymmetric catalysis. Before chemists achieved asymmetric catalysis, many drugs contained two mirror images of a molecule, one of which was curative, and the other sometimes had adverse effects. A catastrophic example is the thalidomide scandal in the 1960s, in which a mirror-image structure in the drug molecule can cause severe malformations in the developing human embryo, and thousands of people were injured.
Now, using organic catalysis, researchers have been able to produce a variety of asymmetric molecules in large quantities in a relatively simple way. For example, they can artificially synthesize candidate drug ingredients that could only be extracted in small amounts from rare plants or deep-sea organisms.
In pharmaceutical companies, this method is also used to simplify the production of existing drugs. Examples of this include paroxetine, which is used to treat anxiety and depression, and the antiviral drug oseltamivir, which is used to treat respiratory infections.
The simplest ideas are often the hardest to imagine
We can list thousands of application cases of organic catalysis, but why has no one put forward this simple, green and cheap concept of asymmetric catalysis earlier? There are many answers to this question.
One is that simple ideas are often the hardest to imagine. Our views are obscured by strong preconceptions about how the world should work, such as the idea that only metals or enzymes can drive chemical reactions.
Benjamin Lister and David Macmillan successfully broke these preconceptions and found an ingenious solution to a problem that chemists have been trying to overcome for decades.
Now, organic catalysts have been able to bring the greatest benefits to mankind.
2021 Nobel Prize in Chemistry: Asymmetric Organic Catalysis